testing the hardness of water pdf|check my water hardness : manufacturers You will use EDTA complexometric titration to determine the hardness of a sample of water brought from your home. Both the total hardness and the individual calcium and magnesium . Resultado da sarada ff dançando quadradinho 59.85K uses, 25 templates - Estamos felizes em apresentar o modelo " sarada ff dançando quadradinho ", uma das escolhas mais populares entre mais de 59847 usuários. Esse modelo oferece 25 estilos diferentes, proporcionando aos usuários uma variedade .
{plog:ftitle_list}
Converse Weatherproof Shoes. Designed to combat the elements, Converse waterproof sneaker boots are made with the worst weather in mind. Available in products like the Chuck Taylor All Star All Terrain and the Chuck Taylor All Star Lugged Winter 2.0,, these waterproof high top shoes are reimagined to stand up to the elements.Include our warm, .
The hardness of water is defined in terms of its content of calcium and magnesium ions. Since an analysis does not distinguish between Ca2+ and Mg2+, and since most hardness is caused by .
When reporting hardness, state the method used, for example, “hardness (calc.)”or “hardness (EDTA).” 2340 B. Hardness by Calculation 1. Discussion The preferred method for determining .You will use EDTA complexometric titration to determine the hardness of a sample of water brought from your home. Both the total hardness and the individual calcium and magnesium .Water hardness is the traditional measure of the capacity of water to precipitate soap. Hard water requiring a considerable amount of soap to produce leather. Scaling of hot water pipes, boilers .
The ions involved in water hardness, i.e. Ca2+(aq) and Mg2+(aq), can be determined by titration with a chelating agent, ethylenediaminetetraacetic acid (EDTA), usually in the form of .Hardness of water is caused by the presence of the calcium ion (Ca2+) and magnesium ion (Mg2+). These ions reach water when the soluble salts of calcium and magnesium are .The hardness may range from zero to hundreds of milligrams per liter, depending on the source and treatment to which the water has been subjected. Standard Methods for the Examination .Water quality is evaluated using a number of parameters, including total ionic content, pH, total dissolved solids, organic compounds, and water hardness. Water hardness is a measure of .
Laboratory Experiment 5: Hardness. Hard waters are generally considered to be those waters that require considerable amounts of soap to produce foam and that also produce scale in .METHODS OF SAMPLING AND TEST (PHYSICAL AND CHEMICAL) FOR WATER AND WASTEWATER PART 21 HARDNESS ( Second Revision ) 1 SCOPE 1.1 This standard prescribes two methods for determination of total hardness, namely (a) Ethylenediamine tetraacetic acetate acid (EDTA) method, and (b) Method based on analytical data andCalcium hardness and methyl orange (M) alkalinity may also be tested. In addition, follow the water testing requirements established by the supplier of the water treatment chemicals where appropriate. Testing parameters for the makeup water include, as a minimum, M alkalinity, conductivity, and calcium hardness. .
PDF | Investigation of Hardness of Water is very important, so it an effort for water technologist for smooth operation and treatment of water | Find, read and cite all the research you need on .concentrations. Total water hardness is usually expressed as the milligrams of CaCO3 equivalent to the total amount of calcium and magnesium present in one liter of water (mg/liter, i.e., ppm). Water hardness may range from zero to hundreds of ppm, depending on the source. The classification of degree of water hardnessChemistry investigatory Project on hard water - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. 1) Hard water is caused by high mineral content, mainly calcium and magnesium ions, that leach into water supplies. These ions interfere with soap and cause scale buildup. 2) Hardness can be temporary, caused by bicarbonates .Laboratory Experiment 5: Hardness Objective: Measure (1) Total hardness and (2) Calcium hardness using dye indicators Background: Hard Water: Hard waters are generally considered to be those waters that require considerable amounts of soap to produce foam and that also produce scale in water pipes, heaters, boilers and
affecting the precision and bias of the test method. Type II water was speciÞed at the time of round robin testing of this test method. 7.3 Ammonium Hydroxide Solution (1+4)Ñ Mix 1 volume of NH 4OH (sp gr 0.90) with 4 volumes of water. 7.4 Buffer SolutionÑ Prepare the buffer solution in three steps as follows: 7.4.1 Dissolve 40 g of sodium .
Testing for Water Hardness Background Info: Hard water has a lot of minerals (Calcium and Magnesium mainly) dissolved in it. Hard water does not mix well with soaps. Because of this, you can test for the hardness of water by looking at the amount of bubbles produced when you mix water and soap. Abstract This paper presents a test method for determining the total hardness in natural and drinking waters using an indicator solution for test titration. The developed method offers a rapid and efficient procedure for assessing the total hardness across a wide range of water bodies. The relative standard deviation of a single result in determining the overall .sample of bottled mineral water. 2. To compare experimental results with the concentrations of the metal ions claimed by the manufacturer. Introduction The ions involved in water hardness, i.e. Ca2+(aq) and Mg2+(aq), can be determined by titration with a chelating agent, ethylenediaminetetraacetic acid (EDTA), usually in the
water hardness testing procedure
📏 Method 3: Hard Water Test Strips. Hard water testing strips offer a quick and easy way to test for hard water at home. A DIY water hardness test works by changing color to indicate the minerals present in the water. You can compare the color of the strip to the color chart, which will help you to determine your water hardness.Temporary hardness of water mg/L (CaCO3 Scale) = Total hardness of water - Permanent hardness of water Observation: The colour of soluble distilled water and R.O water instantly changed into blue while tap water and pond water turned wine red when Ericrome black T was added and therefore after turned blue when titrated against
of different results and does not necessarily indicate that the hardness is present in the water in this form. 2 This test measures total hardness, ie the total content of calcium and magnesium ions in the water. For the specific measurement of calcium hardness and magnesium hardness, refer to the Palintest Calcium Hardness Test.
titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies. The strips contain EDTA andtotal hardness, free residual chlorine, iron, magnesium, mineral oil, boron, cadmium, total arsenic, lead, polynuclear . methods of sampling and test for drinking water. 2 REFERENCES The standards listed in Annex A contain provisions which through reference in this text, constitutecarbonate deposits in the earth, hardness is usually reported as total parts per million calcium carbonate by weight. A water supply with a hardness of 100 parts per million would contain the equivalent of 100 grams of CaCO3 in 1 million grams of water or 0.1 gram in one liter of water. In
Test systems were proposed for the determination of total hardness, acidity, alkalinity, pH, total concentration of heavy metals, and active chlorine in natural, industrial, and potable water by . A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies.Water Hardness Test Kit, Taylor K-0432, Reagent Pack, Buret, Hardness (calcium/magnesium/total), EDTA, 1 mL = 1 mg CaCO₃This very thoroughly supplied test kit contains ten components and is designed for expert-level testing of the hardness level of potable water. . U.S. Government ATSDR Science Corner - 2.5MB PDF; Turbidity test for .
manganese may contribute to water hardness. Water hardness is often defined as the sum of the concentrations of Ca2+ and Mg2+ in water. “Hard” water typically contains high concentrations of Ca2+ and Mg2+, which react with the fatty acids in soap, causing them to precipitate. “Soft” water, such as rainwater or water that has passed . A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies. Hence, water quality evaluation becomes necessary and important to ensure the quality of the water. The water quality can be defined as the chemical, physical and biological characteristics of .This document describes a chemistry project to test drinking water for hardness, iron, fluoride, and chloride. The project was submitted to the CBSE board under the supervision of Mrs. Manisha Sharma by Shiwan Sharma. The objective was to determine the levels of these ions depending on the water source and study the causes of their presence. The document .
Test for total hardness. 5.6.4.1 . Collect a sample of water at the water softener output. 5.6.4.2 . Use an EDTA test or dip-and-read test strips to test for total hardness by measuring the calcium carbonate concentration. 5.6.4.3 . Record the total hardness measured. 5.6.4.4 . If the total hardness is greater than 1 GPG of calcium carbonate .
methods to determine water hardness
Portable Digital Nut Moisture Meter services
Download file PDF Read file. Download file PDF. Read file. Download citation. Copy link Link copied. . These heat-treated samples were then tested mechanically using the Brinell hardness test .
2340 A. Introduction 1. Terminology Originally, water hardness was understood to be a measure of the capacity of water to precipitate soap. Soap is precipitated chiefly by the calcium and magnesium ions present. Other polyvalent cations also may precipitate soap, but they often are in complex forms, frequently with organic constituents, and their role in water hardness may be .Hardness may be expressed in either milligrams per liter (mg/L) or grains per gallon (gpg). A gpg is used exclusively as a hardness unit and equals approximately 17 mg/L or ppm. Those water supplies falling in the hard-to-very hard categories may need to be softened. However, as with all water treatment, you should care-

Portable Digital Soil Moisture Meter services
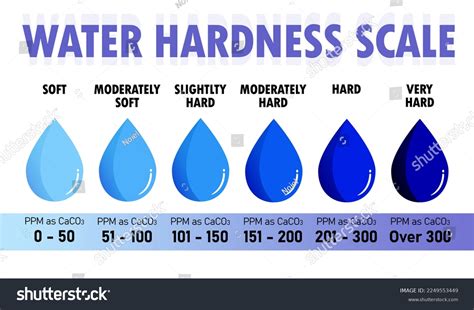
how to test water hardness at home
webApp features: • In-Play Betting – Choose from a vast selection of in-play markets across a wide variety of sports, from football to table tennis to esports. • Cash Out – Sometimes it can be hard to know which way your bet’s going to go and you might be happy to settle for a small profit. • Bet Builder – Combine markets in the same .
testing the hardness of water pdf|check my water hardness